
Low Migration Inks (LMI)

(Written by : N.R. Jayaraman)

Most of the printers in India may not be aware of the developments beyond Quick set and UV drying inks for use on Printing machines or about the legislative norms and regulations in force in the European countries on the use of Inks for printing the wrappers and cartons on food packages. The use of suitable inks to meet the norms and regulations are followed rigorously by the printers and the manufacturers of the food products in the western and European countries since Law enforcing agencies keep stringent control on this aspect. The reason for the norms is to ensure that the print on the packages do not in any way endanger consumer health by the migration of chemicals or other ingredients from the ink on print touching or contacting the food items packed inside the wrappers.
However we are focusing in this article only the aspect of migration of ‘INK’ into the food packs which if allowed will contaminate the food items causing health hazard. Also in focus in this article is how certain measures during the last one decade have been initiated to nullify the said effects. Wherever we mention food package in this article, remember that they are food items such as ready to eat food stuff, beverages and other food items of many types including frozen food items. The issue of hazard due to the ink on food packages gained alarming attention around ten years ago as can be seen from the article published by Shri. Neelakamal Mohapatra in FNM news.com dated Wednesday, 01 June, 2016 in which the author mentions :-
Quote:-
…………Long before the public became aware, a number of scientific publications had already revealed the migration potential of substances present in prints. In the first big migration scandal in 2005, the findings of isopropyl thioxanthone (ITX, a low molecular photo initiator used in UV inks) in baby milk and other liquid foodstuffs were reported, all over Europe and caused several product recalls. This food scandal alerted the packaging chain about the migration potential of substances from printing inks. Due to the complexities involved in designing the composition of the printing inks, the risk of migration of the ink component to the food needs to be considered starting from the selection of the raw materials……….
-Unquote
This is how the attention of the Food and Drug Administration authorities were drawn to bring in legislation in many countries to curb use of hazardous substances in the ink used for printing on food packages and type of packing material to be used for the same. Since then many countries have come out with regulations and norms for suitable ink and packaging material to be used for wrapping or packing food products.
Keeping in view the safety of people and the extent of hazard the chemicals from the ink may cause when the components touch the food items in the packs some European countries through legislation enforced mandatory regulations for the inks meant for printing on the wrappers and cartons of food packages, few of which are cited here:–
(A) Legislation: Swiss Ordinance 817.023.21 for printing inks for food packages.
The legislation states that :
- Printing inks should contain only substances from the inventory list A { (list A* evaluated and list B* non evaluated raw materials).
- Non evaluated raw materials B * can be used when migration can be avoided {(migration < 10 ppb (parts per billion) (* The listed items have not been reproduced in this article)}
The above legislation stipulates that the substances has to be approved before they can be used as ingredients in inks for food packaging. The contents of the regulation contains two lists –List-A and List-B- as Annexure.
The positive list has over 5700 entries grouped as A and B list. The ‘A’ list contains names of substances for which safety assessment dossiers have been submitted, while list ‘B’ contains non evaluated substances which if used will have to meet migration limit of 0.01mg/kg (10 parts per billion) using an officially validated analytical method.
(B) Legislation : EU Regulation 2023/2006. The legislation lays down rules on good manufacturing practice (GMP)* for materials and articles that come into contact with food which includes ink. It states that:
(i) GMP i.e Good Manufacturing Practice means Processes involving the application of printing inks to the non-food contact side of a material or article.
(ii) Printing inks applied to the non food-contact side of materials and articles shall be formulated and/or applied in such a manner that substances from the printed surface are not transferred to the food-contact side either:
- Through the surface
- By set off in the stack or the reel in concentration that leads to the levels of the substance in the food which are not in line with the requirements of Article 3 of Regulation (EC) No 1935/2004.
(iii) Printed materials and articles shall be handled and stored in their finished and semi-finished state in such a manner that substances from the printed surface are not transferred to the food-contact side either :
- Through the surface
- By set off in the stack or the reel in concentration that leads to the levels of the substance in the food which are not in line with the requirements of Article 3 of Regulation (EC) No 1935/2004.
(iv) The printed surfaces shall not come into direct contact with food.
- Effective quality system be maintained
- Starting materials in compliance with pre established specifications
- Migration or invisible set off of components of printing inks shall not exceed the limits
- No direct contact of printed surface with food.
(C) In US, the Food and Drug Administration called FDA regulates the material which will come into contact with food. Naturally it applies to ink on the food pack containers and wrappers. There is a basic assumption that any material used in food contact applications (including ink) will become part of the food unless documented testing proves otherwise. The FDA provides a list of approved materials in title 21CFR (code of Federal Regulations).

The Inks and coatings that do not have direct food contact are not regulated, as long as there is a functional barrier between the food contact side and the ink or coating, and the inks and coatings do not migrate to the food contact side during various steps in the process. It is responsibility of the packaging manufacturer to determine if the construction meets the definition of a functional barrier (Ref: Adherence to high standards for food packaging inks a must for consumer safety by Shri.Sanjeev Bansal | Mumbai dated March 20, 2014).
(D) Though India has BIS standards for manufacture of printing inks for use on food packaging, it is only voluntary. However Bureau of Indian Standards (BIS) have issued guidelines for manufacture of printing inks used for food packaging vide IS: 15495:2004: May 2010 under code of practice** to be followed. Unfortunately, this is again not being enforced to its full potential. It is only voluntary standard for adaption even though India has been a significant importer and exporter of food.
( **The gist of guide lines given in IS standard is reproduced at the end for information).
As the legislation and norms began to be issued in many countries for the use of non hazardous type of inks for printing on the food packs, some of the ink manufacturers came out with Low Migration Inks and Safe inks for food packages which complied to the provisions of legislation in this issue.
What is meant by migration of inks, how important is this aspect for the inks used on food packs and whether the Low Migration Inks are in meeting the norms of the legislation ? Let us analyze the issue one by one.
Migration of ink means transfer of certain quantity of component from the ink through the walls of the packing touching the food, or vice versa.
The migration of ink through the surface of packing material on food packages takes place in three to four ways as indicated below. Such migrated traces of the ink may not get detected in odour and taste tests when the food is consumed, but can be identified only by sensitive chemical analysis.
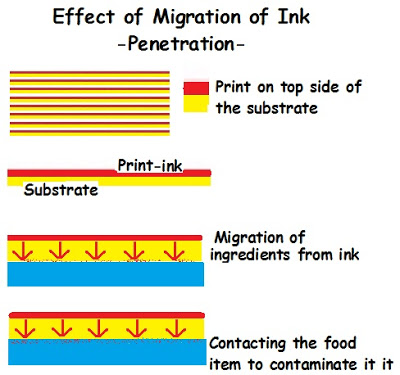
- Migration by Penetration: Migration of the ink component of low molecular weight penetrate into the substance from the printed side to reach the non printed side i.e inside the pack thus contacting the food items.
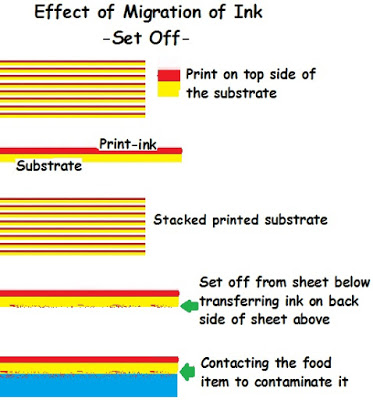
- Migration by Set Off: While printing, sometimes the wet ink print from the substrates that follow may leave some impression on to the back side of the previous printed sheet above and it is called set off. Since the non printed side (Back side of the substance) forms the inner wall in the pack, the residual ink ingredients (set off) stuck to it comes into contact with the food item inside.
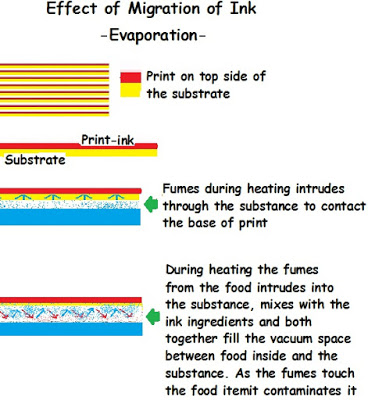
- Migration by evaporation : During heating or baking process of frozen food packed in the microwavable packs, the volatile substances in the enclosed air space inside the packaging can get transferred to the food via the fumes or gas phase and may cause negative effect on the smell or taste of food items. Some components of the ink that has penetrated into the inner wall of the substance may also mix up with the fumes in the vacuum space to contaminate the food items. Even changes in the environment conditions may cause this reaction.
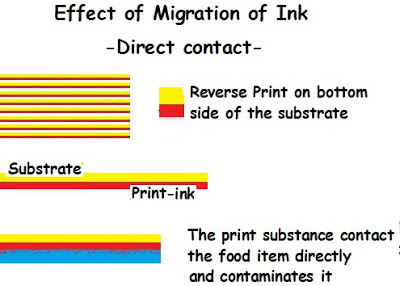
- Migration by Direct Contact: While printing, sometimes the print is made on the reverse side of the transparent packing substance in such a way that the print can be seen from above. Since the print on the wall inside touches the food item directly, the ink ingredients comes into contact with the food item inside.
How is the extent of migration of ink indicated or measured?
The level or concentration of ingredients of ink migrants permitted by law (internationally) is usually expressed in terms such as Mg/Kg (measure per kg) or PPB (parts per billion) of the food content. Someone may refers the migration as 6 mg/kg, then it can also be viewed as as 6 ppm or 6000 ppb. For example if after testing a ingredient or the migrant substance of ink is addressed as 74 mg per kg, that means that for every kilogram of ink, 74 milligrams of migrant product may migrate. So if the measurement of the component is 4300 mg/kg, there will be 4300 mg of the said component in every kg. The higher the number, the higher will be the level of migrants per kg.
What is the upper limit prescribed for migration of ink?
Is legislation or norms or regulations prescribe upper limits for migration in respect of Ink ? No, there are no clear indications except in Swiss regulations. Remember one basic aspect. Ink alone is not solely responsible for the process of migration. It is one amongst many other factors. The amount of migration depends upon several factors such as the substance used for packing, nature of packing, type of food item and the conditions of their use besides the type of ink used on print. At present, no regulation exists that specifically deals with the formulation of inks meant for foodstuff packaging even though migration limits have been prescribed for the substances used like the packaging materials used. Read the following interesting information:
Quote:-
……Printing inks are currently not specifically regulated under European Law, though substances used in food contact materials shall generally not endanger human health (Article 3, EC 1935/2004) and some printing inks are regulated under the plastics regulation EC 10/2011 act. In consequence, most printing inks continue to lack toxicological evaluation and specific migration limits (SMLs) in Europe. Yet, the Swiss Ordinance on printing inks contains positive list of substances for the printing inks ….. (Ref:- Printing ink exposure from FCM significantly underestimated – written by Charlotte Wagner dated October 30, 2013 in Food Packaging Forum)
– Unquote
To our knowledge except Switzerland, no other country has stipulated specific norms for the use of Inks for food packaging. It is learnt that the Swiss Federal Department of Home Affairs (FDHA) recently adopted an amendment to the Ordinance dated 23rd November 2005 issued for use of Materials and Articles (SR 817.023.21) relating to packaging inks. This amendment, introduced on 7th March 2008 and coming into force on 1st April 2010, Article 26g, details the requirement to state that only permitted substances should be used in the manufacture of Packaging Inks. Permitted Substances are defined as those which are listed in Annex as ‘Lists I’ and ‘List-II’ and in ‘Annex 6’. (Ref: http://www.eupia.org/index.php?id=30)
In view of stricter Legislation and mandatory norms enforced by several nations, the printers around the world have begun to use alternate ink other than the standard inks for printing on Food packages in order to comply with the regulations and norms meant for printing inks used on food packages and in this direction the ink manufacturers too have come out with Low Migration Inks. With no global legislation available and terminology not definitely defined, ink formulation for food packaging remains a challenge for suppliers who work closely with customers to ensure compliance with various guidelines and the safe production of printed food products.
What is the basic difference between Standard and Low Migration Inks ?
Standard Inks being low in molecular weight have lower viscosity. The drying components in them have the potential to migrate. The Low migration inks replace these properties with high molecular weight and evaluated substances. In addition to food, many pharmaceuticals, personal care products, child and baby care products, beverages, and tobacco products require inks with low-migration properties.
What is Low Migration Ink ?
The term Low Migration Inks means the ink on the print will not quickly intrude through the walls of the packaging material to contact the food stuff packed in. The qualities of such inks are that they are made with only with such components which do not have the tendency to migrate, or move away from the top layer of the pack to intrude into the other side of the pack. The extent of migration of those inks will be just hold on to the top layer of the outer wall. In short, to qualify as Low Migration Ink, they must not have any migratory chemicals as their component which if allowed would affect the appearance, flavour, odour, taste, or the safety of the product placed inside the pack. The migration of printing inks or permissibility to intrude also depends on the properties of the substance on which they are printed. The materials may be of any type such as Paper, board, plastic or other polyester substrates etc. While paper based carton and board or even thin plastic substrates have high level of permeability, the glass has no permeability and therefore any ink can be safely used if glass base material is used as packaging material for food items.
Since the migration depends upon the material which is manufactured using different types of raw materials by each manufacturer, the ink suppliers will have to ensure that the medium on which the ink is to be used is well analyzed for manufacturing suitable ink before the ink supply is made. This aspect gains importance where brand protection issue comes to the fore and only specific material is used as food package material and therefore customization of specific ink to meet the provisions of non migration tendency is quite easier to produce.
For example the behaviour of Low Migration Inks used on one kind of Card board may not effectively give same result on another Card board which may have been manufactured using different other raw material. Low Migration Inks used on Cardboard or Polyester may not work effectively on different other plastic based carton. Therefore the best effect of Low Migration can not be obtained by ink alone, as other factors also come in to play which includes pack and prints design; how the ink is applied and dried; ink coverage; the substrates used and storage conditions. Regardless of how an ink is formulated, the ink alone cannot be considered low-migration until it’s actually applied to a specific substrate.
Colorcon’s No-Tox® Products Division manufactures the safest line of U.S. Food & Drug Administration (FDA) regulated printing inks. These inks are used exclusively for printing applications involving direct and indirect contact with food, pharmaceutical or medical products. No-Tox® printing inks are ideal for packaging that directly contact food and confectionery items. Sun Chemicals, Sicpa and Siegwerk are in forefront in manufacturing Low Migration Inks.
The Low Migration Inks have also been introduced for Inkjet printing. Agfa’s low-migration (LM) UV-curable inkjet inks are designed primarily for printing directly on food packaging and on labels, blister foils, lids etc for food and pharma packaging applications. These low-migration inks are based on specific chemical formulations, compliant with European guidelines on food package printing.
The UVAFLEX® Y71 Series of inks manufactured by Zeller+Gmelin GmbH & Co. KG has a low-migration formulation and is therefore suitable for printing of primary packaging for food. The UVAFLEX® Y71 Series is a new developed low migration ink series for flexible packaging and labels.
Many firms across the globe specialized in manufacturing inks have come out with Low Migration Inks suitable to print on for food packages.
Some of the known firms manufacturing inks suitable for food packages are :
- Siegwerk, India
- Sicpa, Switzerland
- Sun Chemical, UK
- USAINX International, USA
- Flint group, Germany
- EuPia, Belgium
- Gallus, Switzerland
- Marabu GmbH & Co, Germany
- HP Indigo
- Zeller+Gmelin UK Ltd
- Frimpeks® UK/Germany
- Huber group, Germany
The code of practice stipulated in IS 15495: 2004 standard for manufacture of Printing Inks for food packages are briefly mentioned below:
(1) For for printing external food wrappings, where there is a barrier in the form of another wrapper between the printed surface and the food:- The very low mass of the ink generally used to print such a packing and the remoteness of ink itself from the food make any additional safeguards unnecessary. The components in printing ink need to comply to exclusion list given in Annex A which contains list of Pigments and compounds based on antimony*^, arsenic, cadmium, chromium (VI), lead^ mercury and selenium; various Dye colorants, limits of heavy metals contained in pigments and dyes, solvents etc.
(2) In case the printed ink film is deliberately applied to the surface intended to be in contact with food, it is possible that migration of some ingredients into the food may occur and therefore, the printing ink for such a purpose shall have to be formulated with materials which are permissible as food additives and comply with the appropriate regulations of the Government of India.
(3) The over coating of printed matter with a varnish to provide a functional barrier between the printed side and the food may not, under all conditions, prevent migration of some ingredients from the ink into the food and therefore, may not prevent contamination. It is, therefore, necessary that inks for immediate food wrappings must be applied to the outside of the wrapper. The wrapper itself shall form a functional barrier between the printed surface and the food.
(4) The ink film on a wrapper is generally extremely thin and consequently, the total quantity of ink involved is very small. However, in order to impose the safeguard, inks for immediate food wrappings shall be formulated with materials other than those known to be toxic and shall not contain material listed in Annex A (Details as given under Sr 1 above).
(5) The immediate food wrappers shall be printed in such a manner that set-off in the printing process is avoided. This is necessary to ensure that the surface of the wrapper in contact with food is free from printing ink.
(6) The materials and articles in contact with food, that is, food packages or wrappers shall be so manufactured that under normal or foreseeable condition of use, they shall not transfer their constituents to the food in quantities which may endanger human health.
(7) However, if it is necessary for the printed surface to be in direct contact with food, the guidelines prescribed in 4.3,5 shall apply and the printing inks shall have to be formulated with materials, which are acceptable as food additives under the appropriate regulations of the Government of India.
(8) In case of printed films or coupon inserts for dry granular foods, printed inks shall be formulated in such a way that there is no reasonable risk of the print migrating onto the food. In general, requirements of 4.3.1 and 4.3.2 apply there.
(9) Printing inks for disposables shall be formulated with materials necessarily excluding those covered in Annex A or those, which are otherwise known to be toxic. As far as possible and practicable, the printing ink manufacturers shall ensure that inks are formulated in such a way as to avoid migration of dyes or other coloring agents, liable to bleed under the expected conditions of use, onto the food.









Recent Comments